Protein Expression in Bacteria
We have extensive expertise in vector design. We also understand how the quantity and quality of expressed protein depends not only on vector elements, but also on environmental factors such as temperature and media components. Below are examples of the tools we use to optimize the expression of your protein in bacterial expression hosts.
Vector Elements
Promoters
ATUM uses several different inducible promoter systems.
- The rhamnose-inducible promoter combines high expression levels with precision of control.
- The T7 system requires special host cells containing a chromosomal copy of the T7 polymerase under control of the lac promoter. IPTG induction expresses T7 polymerase, which then transcribes any gene downstream of a T7 promoter.
- The IPTG-inducible T5 promoter consists of a strong constitutive promoter flanked by lac operator sequences and works in any strain of E. coli.
- The PhoA promoter does not require expensive or metabolizable inducers, but auto-induces once the cells have depleted the phosphate from the media.
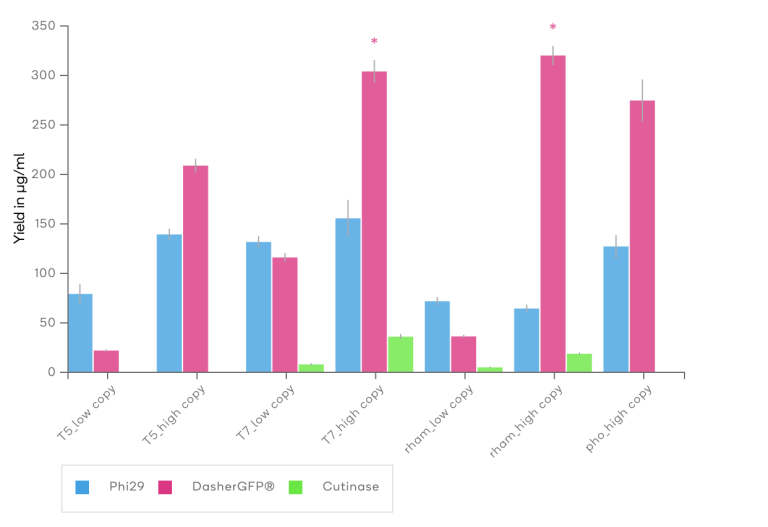
Promoter Properties
Expression using a specific promoter can be further controlled by the copy level of the origin of replication. In particular, uninduced expression levels of toxic proteins can be further reduced by using low copy versions of the vectors.

Signal Sequences
In gram-negative bacteria, proteins tend to be poorly secreted and are often directed to the periplasm. The periplasm provides a different environment to the cytoplasm, most significantly the periplasm is oxidizing and allows disulfide bond formation. During bacterial protein expression, the effectiveness of a signal sequence in directing the protein to the periplasm varies depending upon the protein being expressed.
Open Reading Frame
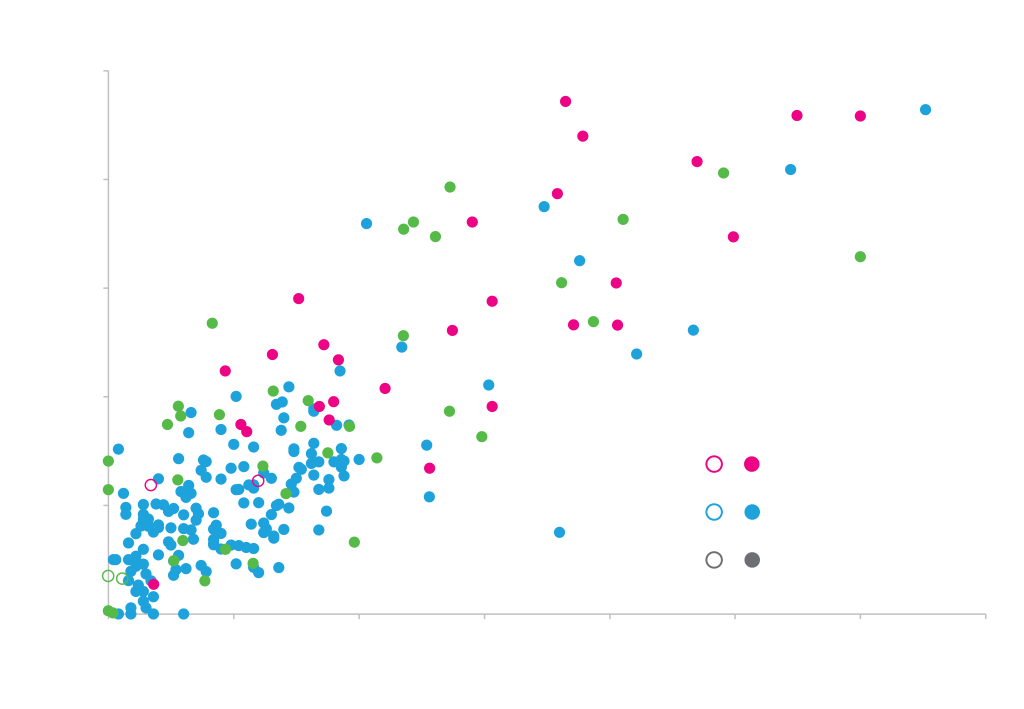
Our earliest work in developing our GeneGPS® design platform was performed in E. coli. From E. coli codon optimization, we have subsequently applied GeneGPS® to optimize genes for expression in a range of bacterial hosts including Bacillus and Clostridium. By designing and testing hundreds of genes and modelling the data, we are able to identify the critical sequence parameters that most strongly influence expression. The graph below shows a strong correlation between our gene optimization model predictions and measured gene expression levels: this is the same model that our backtranslation algorithms use to design genes for bacterial protein expression.
Solubility-Enhancing Fusions
Proteins expressed in bacteria do not always fold well and can aggregate in insoluble inclusion bodies. ATUM uses several different fusion tags during bacterial protein expression to improve the solubility of your proteins. Protease cleavage sites between the tag and your protein allow the removal of the tag from the desired protein product.
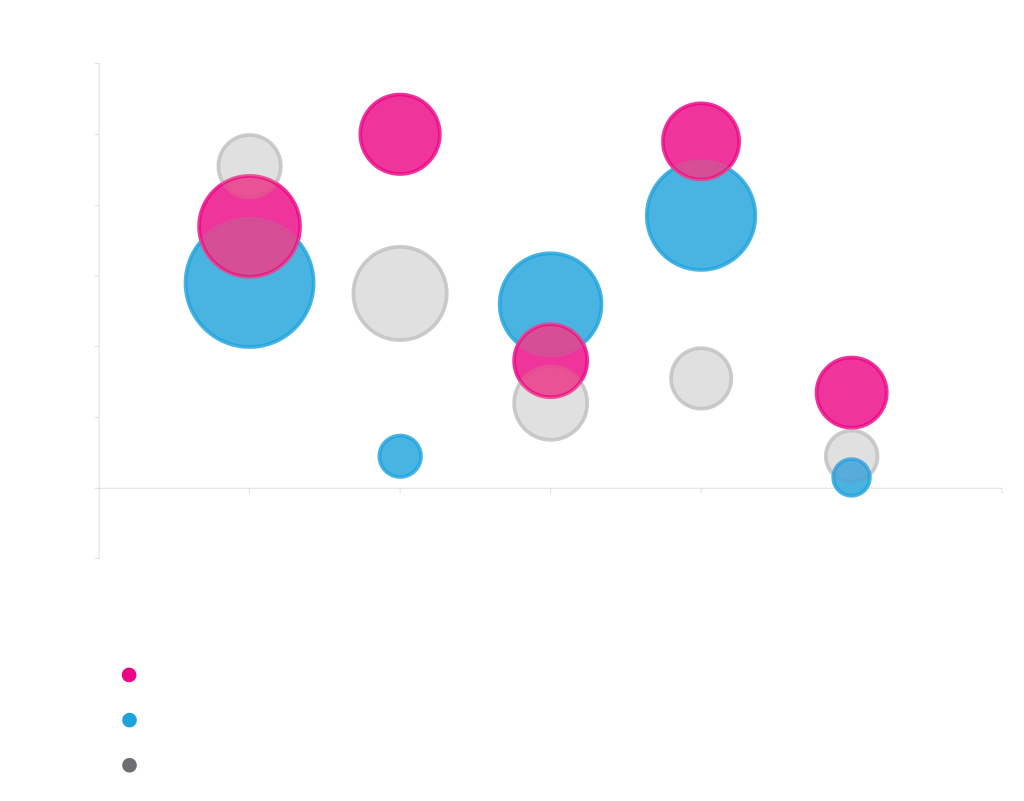
Effectiveness of solubility-enhancing fusions is protein dependent
Effect of different tags on the yield and solubility of three different proteins produced in E. coli. The His tag is an affinity tag but does not generally affect solubility. It is included here to show the solubility of the proteins in the absence of solubility-enhancing fusions.
Protein Expression Optimization
In addition to vector elements described above, ATUM further optimizes protein expression using a range of conditions including temperature, induction level, lysis conditions and special media additives, for example riboflavin for FAD proteins and limiting metal cofactors. ATUM also offers Expression test services to determine the best vector, growth and induction conditions for your protein.