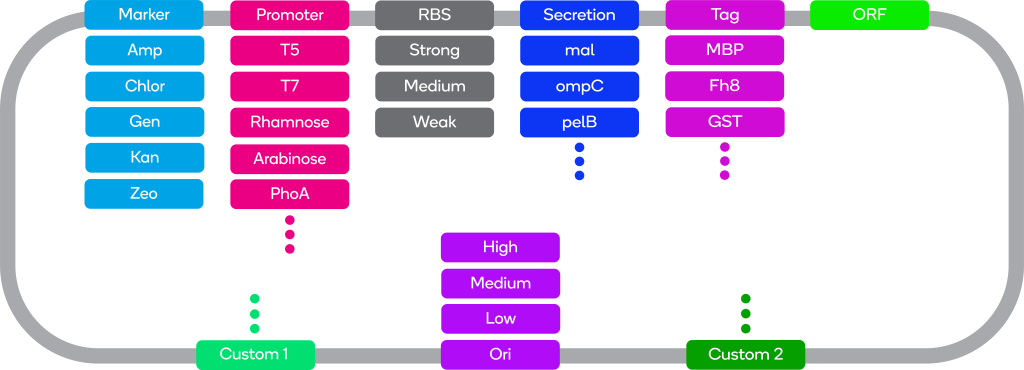
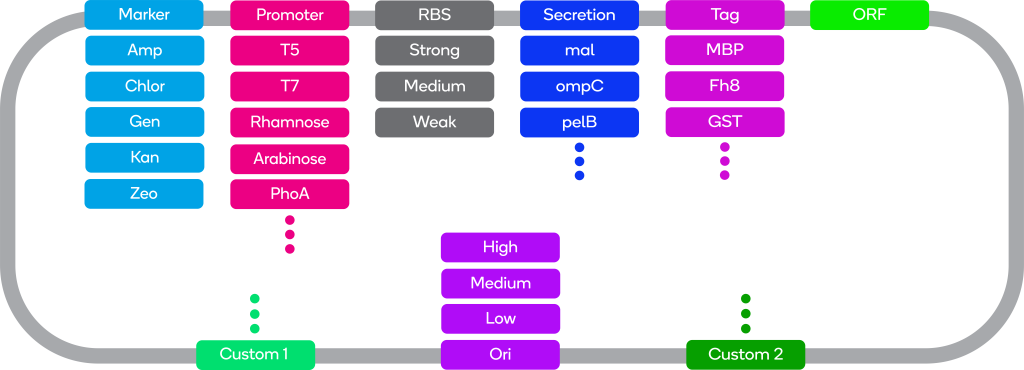
ATUM offers a range of bacterial expression vectors. Combinations of different promoters, selectable markers, and copy numbers enable you to choose a vector suited to your expression system.
YIELD
Not sure how your protein will behave? Test your gene in ATUM’s ready-to-use vectors with a variety of properties most likely to increase expression.
SPEED
ATUM offers pre-selected Expression or Secretion panels, or you can create your own to quickly explore the properties you think are most important.
PRECISION
Combining promoters with different strength ribosome binding sites and origins of replication provides an excellent range of induced and uninduced expression levels.
Promoters Overview
ATUM offers rhamnose, IPTG (T5 and T7) and PhoA-based inducible bacterial expression vectors. Combining these with different strength ribosome binding sites and origins of replication provides an excellent range of induced and uninduced expression levels.
Promoter Properties

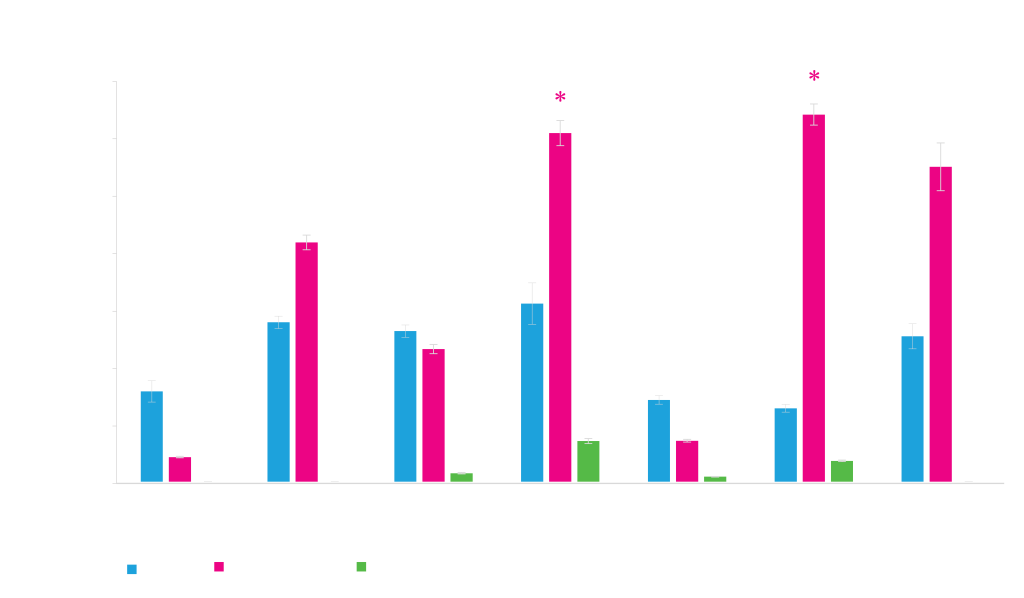
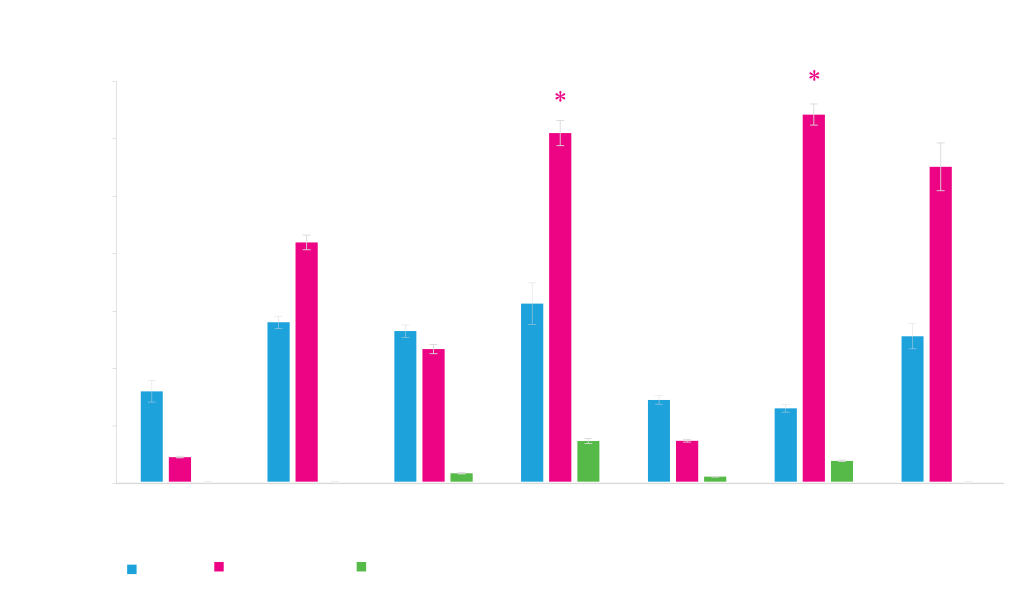
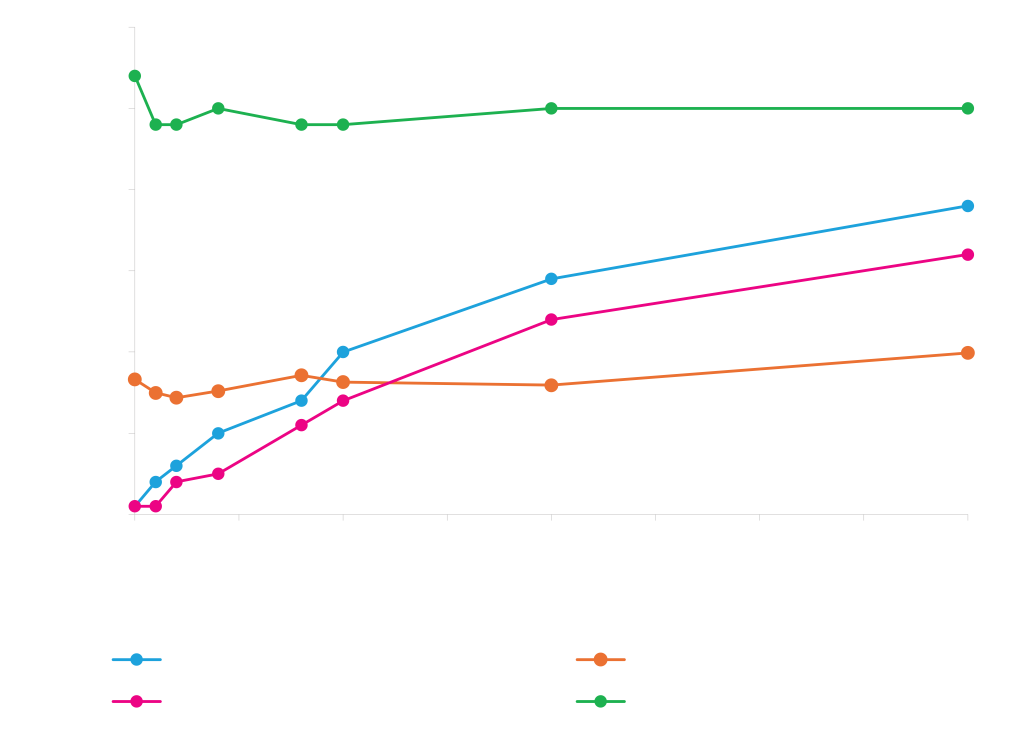
Protein Expression from Different Promoters
Different promoters work better for different proteins. Expression of three proteins, phi29 (~61 kD), cutinase (~22 kD) and DasherGFP (~26 kD), under control of different promoters, T5 and T7 (IPTG-inducible), rham (rhamnose-inducible) and phoA (inducible by phosphate starvation) is shown. Expression values shown on the y-axis are measurements of expressed protein band densities from a SDS-PAGE gel using BSA as standard.
*DasherGFP measured fluorescence is lower with T7 and rham expressed GFP, indicating that a fraction of total protein is incompletely folded protein.
Independent Expression Using Two Orthogonal Promoters
Choice of promoters and inducible systems allow orthogonal expression of two proteins in a single strain by using a two vector system for induction control of each individual protein. Choose from vectors with promoters that are IPTG-inducible (T5 or T7), rhamnose-inducible, arabinose-inducible, or inducible phoA.
Orthogonal Expression of YFP and CFP in a Two Vector System:
- Cloning of YFP and CFP into two pDAUGHTER vectors – rham_YFP and T5_CFP
- No interference observed – expression is exclusive
- Comparable expression with Rham and T5 promoters
Tags Overview
Affinity tags are epitopes that when fused to either the C- or N-terminus of a recombinant protein are highly efficient tools for purifying proteins from crude extracts. This enables highly selective capture and circumvents multistep purification processes. In addition, the tags can be used for detection and may also help enhance protein expression.
Solubility-enhancing tags are generally large peptides or proteins that increase the expression and solubility of fusion proteins. No single fusion tag can increase the expression and solubility of all target proteins.
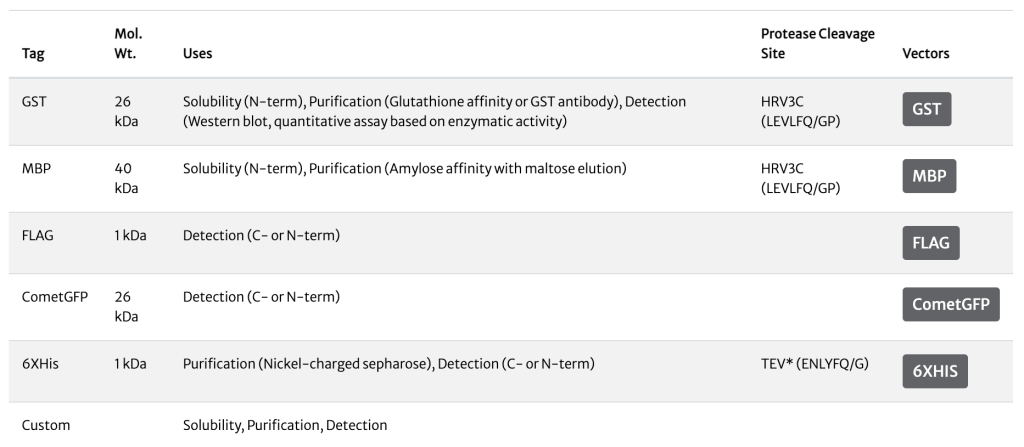
Available Tags
N-terminal fusions can also help with expression as they can iron out any secondary structure that interferes with expression.
An integral part of fusion tag choice is the method for removing the tag after purification. This step almost always involves using a protease to cleave a specific peptide bond between the tag and recombinant protein. ATUM offers the tobacco etch virus (TEV) protease with a 6XHis tag and the HRV3C protease with a GST tag to facilitate removal of the enzyme post-cleavage.
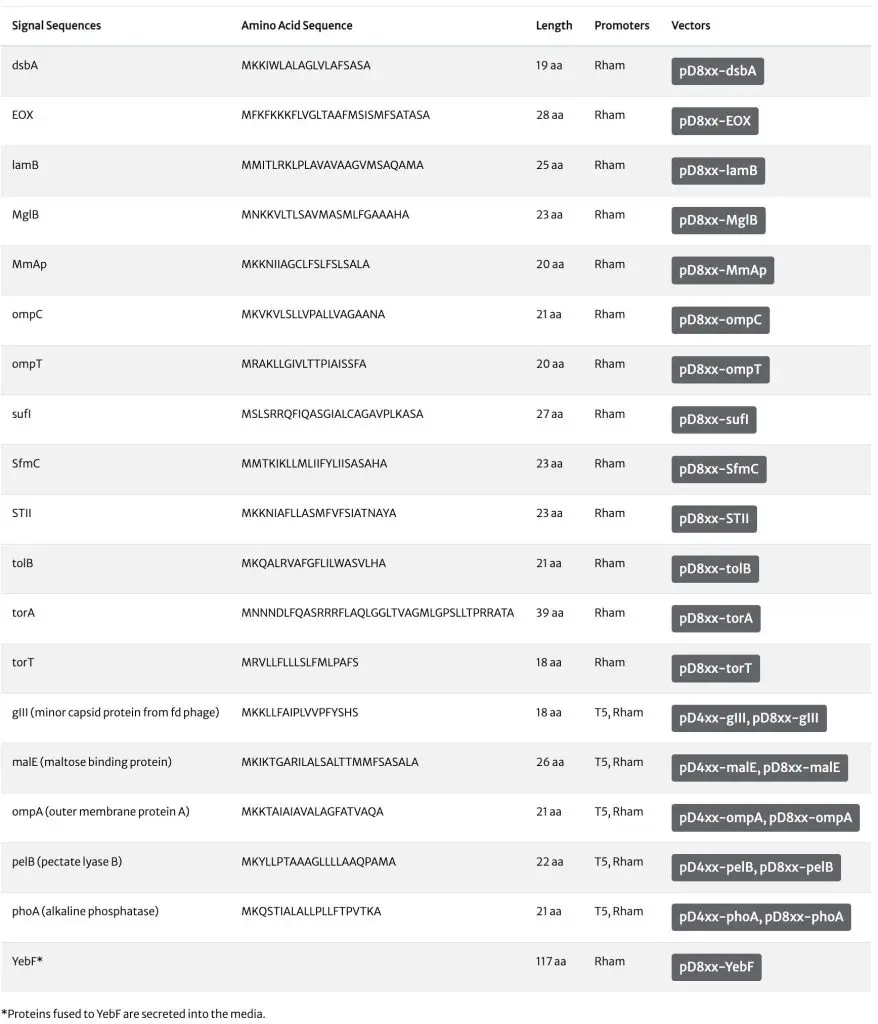
Secretion Signal Overview
Why secreted expression?
Production of secreted recombinant proteins using E. coli rhaBAD promoters offers several advantages compared to cytosolic production, such as simplicity of purification, avoidance of protease attack, and a better chance of correct protein folding. Since the use of a post-translational or co-translational export mechanism is protein-specific, vectors with different secretion signals allow for the selection of the translocation pathway best suited for a given recombinant protein. Note: ATUM recommends the use of vectors with rhamnose-inducible promoters for secreted protein expression. There are many distinct advantages to the rhamnose secretion vectors:
- Expression of protein is tightly tunable with rhamnose concentration thus allowing you to modulate protein expression, so as to avoid overwhelming the secretion machinery.
- Rhamnose vectors are available with a wider selection of 18 secretion signals spanning the Sec, TAT, and SRP translocation pathways.
Vectors are also available with YebF to enable secretion into media
ATUM vectors with different secretion signals allow selection of the secretion signal best suited to each protein. Parallel processing of multiple pDAUGHTER secretion vectors allows a quick and convenient method for selecting the signal(s) which provides the highest levels of secreted expression. While different secretion signals have been used, individual levels of secretion may vary and depend upon the recombinant protein being expressed. There is no general rule to selecting a secretion signal to guarantee successful secretion for a given recombinant protein, as efficiency of secretion depends on host strain, signal sequence and type of protein.
Secretion Signals
Use the ATUM Expression Test to determine the best vector for your construct. ATUM will clone and test your construct in a full bacterial vector panel to quickly determine expression levels in E. coli.
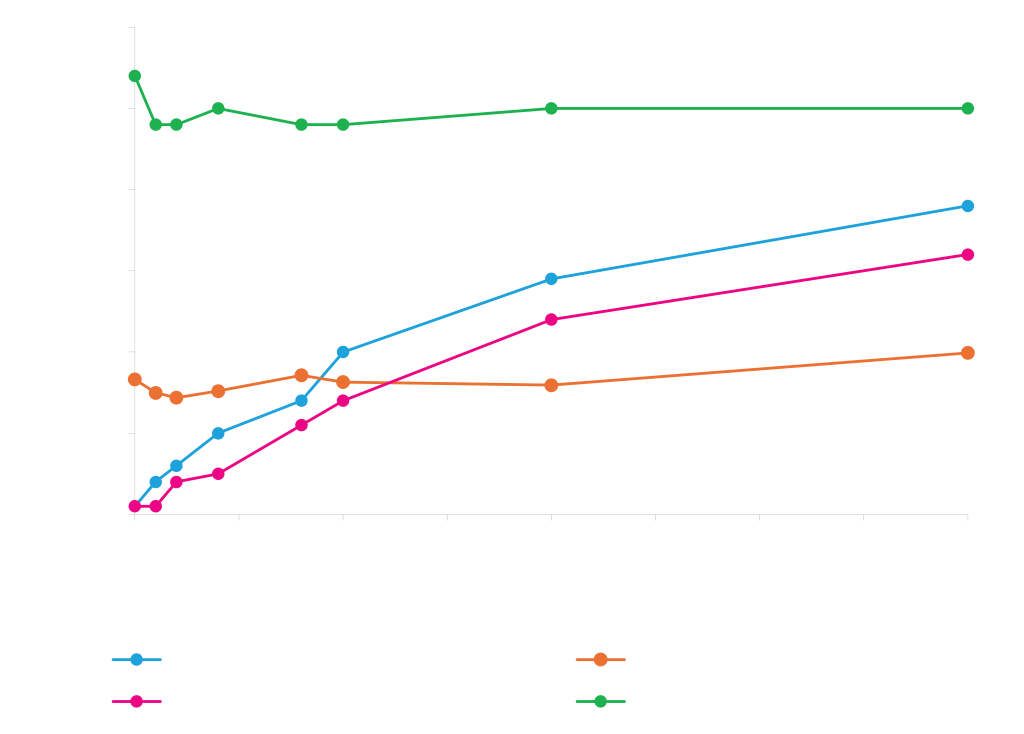
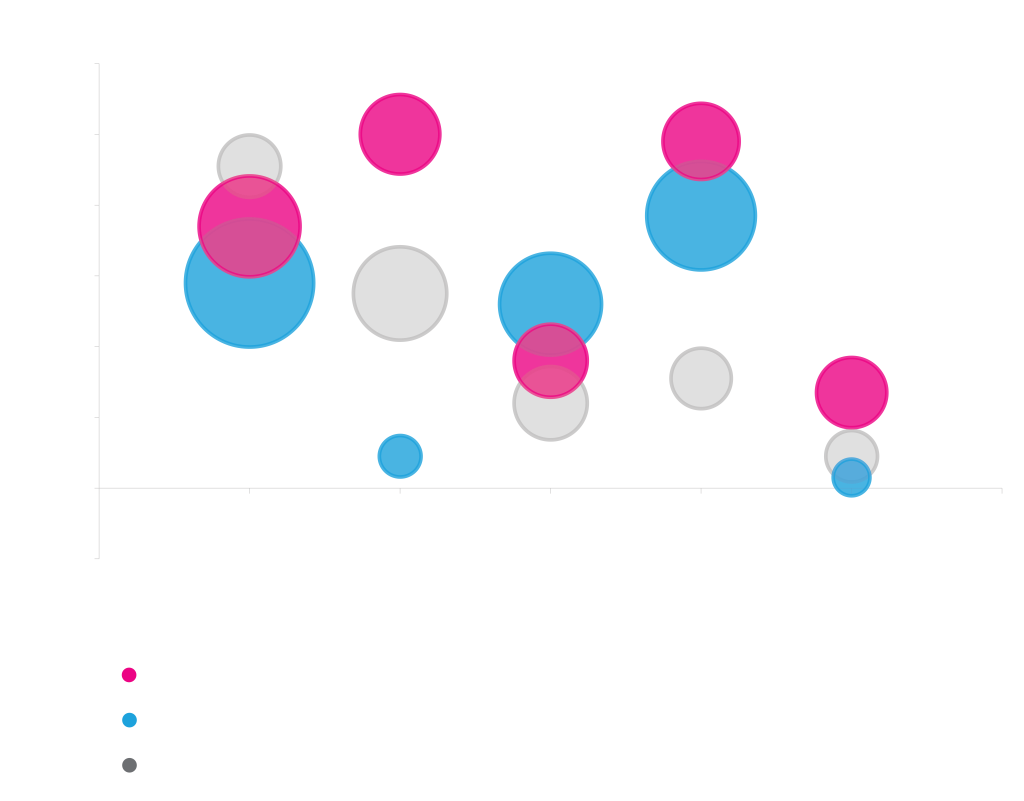
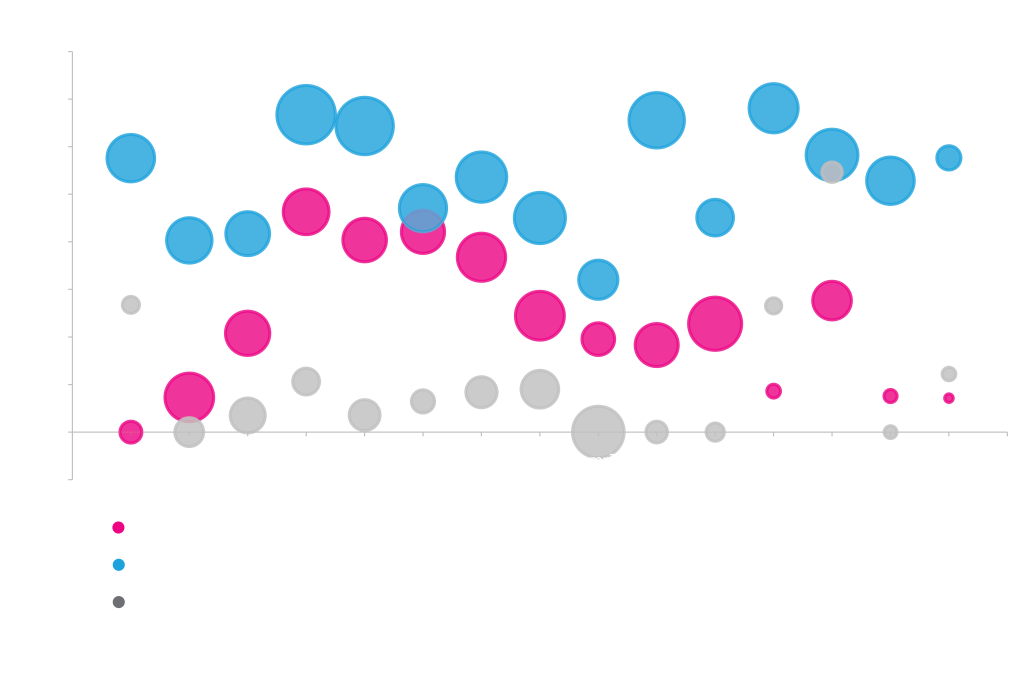
Expression with Secretion Signals
Different proteins prefer different secretion signals. Periplasmic expression of MBP, Cutinase, and Alkaline phosphatase from rhamnose vectors with different secretion signals. Total cellular protein levels are represented as circle diameter. Soluble protein in the periplasm is shown on the y-axis. Protein was quantified by densitometry of stained acrylamide gels.