Electra System
SPEED
Fast and easy gRNA design using ATUM’s design tool that minimizes off-target effects using ATUM’s scoring algorithms. ATUM will clone your gRNAs into the CRISPR construct of your choice and send you ready-to-transfect plasmid.
SPECIFICITY
Easy design of two tandem gRNAs for NickaseNinja vectors to enhance specificity.
PRECISION
The design tool enables suppression of off-target effects.
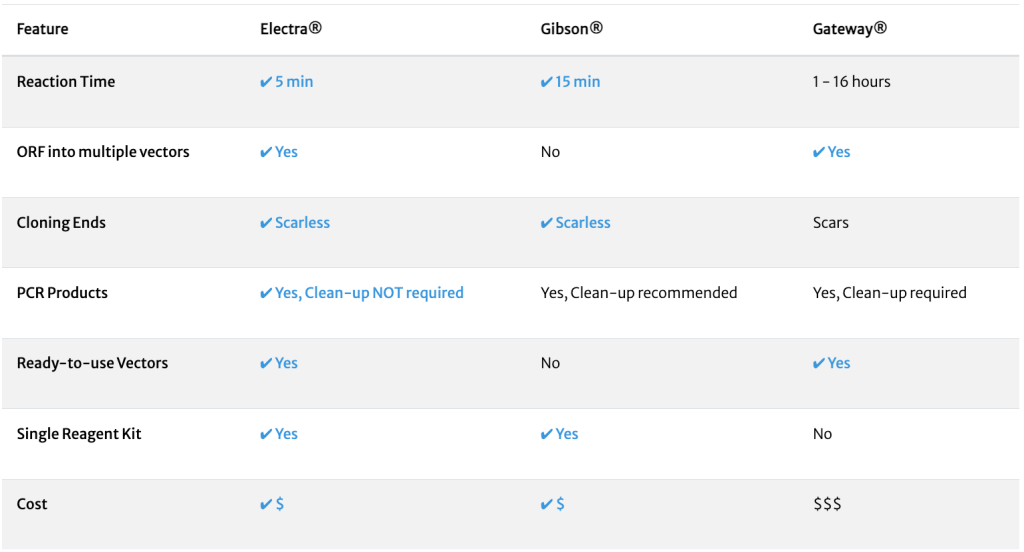
Electra Vector System
Electra Advantages
- Easy – 1 tube, 5 minute reaction
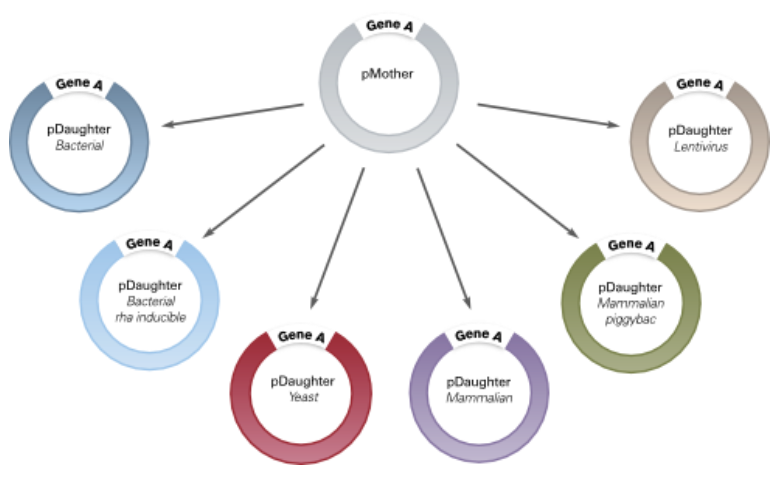
- Universal – any ORF cloned into any Electra vector. Quickly move ORFs from MOTHER vector to multiple DAUGHTER vectors
- Scarless – always in frame with no nucleotide scars
- Choice – Large selection of vectors for multiple hosts with fusions, bicistronic expression, promoters, RBSs, and more
- Convenient – clone your genes using the Electra Cloning kit, or have ATUM do the work for you
- Original reference; Whitman et al., Genetic Engineering News 2013 (Vol. 33, No. 12)
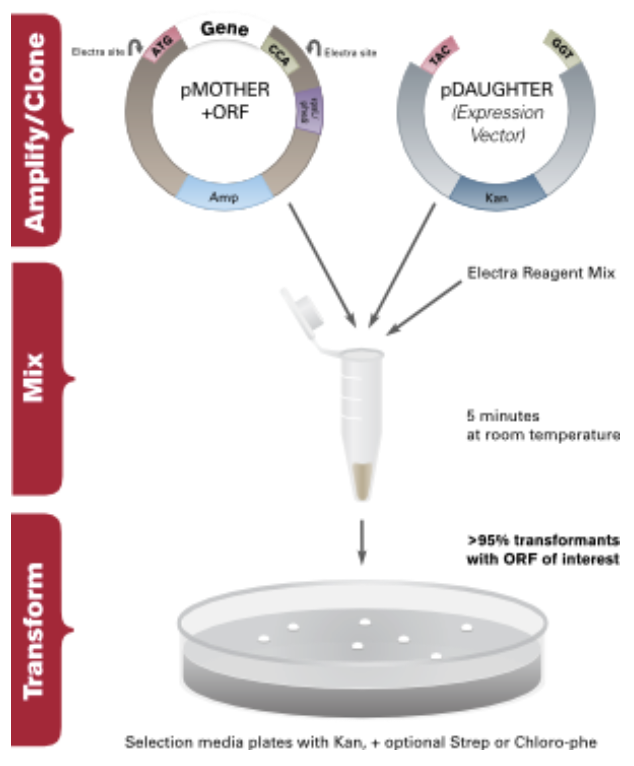
How Electra Works
ATUM has developed a simple, one-tube, universal cloning process that is performed in a 5 minute bench-top reaction with the fidelity of a restriction based cloning system. The Electra system uses the type IIS restriction enzyme SapI, which recognizes a 7bp non-palindromic recognition sequence and leaves a 3bp 5’ overhang after digestion. We have developed a collection of bacterial, mammalian and yeast expression vectors that provide a quick and efficient way to clone a gene of interest under control of various elements and are available with optional C- and N-terminal tags and/or fusions. Any vector can be easily “Electra-fied” (converted to function as an Electra vector), and ATUM will assist anyone who wishes to do so.
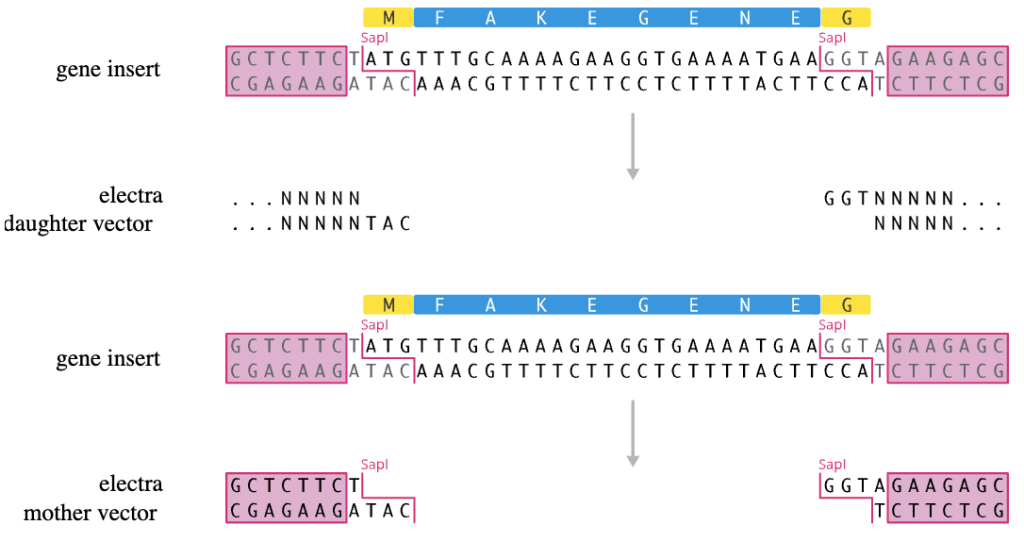
Electra Protocol - PCR Primer Design
The Electra system uses the type IIS restriction enzyme SapI. SapI recognizes a 7bp non-palindromic recognition sequence and cuts outside of the recognition sequence, leaving a 3bp 5′ overhang after digestion. ORFs in a pMOTHER vector can be excised with SapI, resulting in ATG and GGT overhangs. Alternatively, PCR can be used to generate ORFs with the correct overhangs. These ORFs can then be easily cloned into pDAUGHTER vectors, which are provided linearized with the corresponding overhangs.
We recommend you add the following ends to your primers, as these contain the Electra sites to clone directly into Electra vectors. Add 15-20 bp of your ORF to the 3′ primer end to amplify your ORF and have it compatible with any of the Electra MOTHER or Electra DAUGHTER expression vectors.
- Forward primer with ATG start codon on primer:
- 5′-TACACGTACTTAGTCGCTGAAGCTCTTCTATG….(ORF beginning after ATG start codon)….
- Reverse primer (same as above):
- 5′-TAGGTACGAACTCGATTGACGGCTCTTCTACC….(ORF Reverse Complement)….
Note: For pD861 vector series with secretion signals, a forward primer with GCA codon is used.
- pD861-XXX forward primer with GCA start codon on primer:
- 5′-TACACGTACTTAGTCGCTGAAGCTCTTCTGCA….(ORF beginning after GCA codon)….
- Reverse primer (same as above):
- 5′-TAGGTACGAACTCGATTGACGGCTCTTCTACC….(ORF Reverse Complement)….
Additional Info
A PCR mixture can be directly cloned into pMOTHER or pDAUGHTER vectors using the Electra reagents. However, if your PCR reaction shows multiple bands by gel analysis, it is very likely that some fragments other than your gene of interest will also contain SapI ends and may be cloned into your Electra vector. Additional screening of colonies will be useful to identify clones containing your gene of interest.
Your ORF must NOT contain any SapI recognition sites, as the Electra cloning process utilizes the type IIs enzyme SapI.
Cloning Protocol
*From the Electra Cloning Kit
Donor DNA can be PCR product or Mother vector DNA. Mother (pM) and Daughter (pD) vectors are available as linearized vectors, 10 Rx at ~20ng/µl
- Combine components as listed in table above in a single 1.5ml tube. Note Daughter Vectors come pre-linearized from ATUM.
- Incubate 5 – 20 minutes at room temperature.
- Transform 2 µl of each reaction into competent cells*. Note cells used at ATUM are streptomycin resistant cell lines.
- Plate onto LB plates with selection antibiotic alone, or with selection antibiotic and 100 µg/mL streptomycin (Teknova #L1148 Kan+strep, Streptomycin resistant strain such as DH10B is recommended if using pMother with rpsL counter-selection), or YEG plates with selection antibiotic and 16mM p-chloro-phenylalanine (Teknova #Y5700 (Kan+chloro-phe) or #Y5705 (Amp+chloro-phe)).
- Incubate overnight at 37°C.
ATUM has tested the Electra vectors in DH10B E. coli cells. We recommend the use of competent DH10B cells for all Electra transformations.
Cloning from pMOTHER plasmids
To determine the time course and selectivity of a standard Electra reaction.
- pMOTHER constructs: a yellow fluorescent protein gene (KringleYFP) was cloned into two pMOTHER vectors:
- pMOTHER264 – pUC bacterial origin, Amp resistance, rpsL counter-selection marker
- pMOTHER268 – pUC bacterial origin, Amp resistance, PheS counter-selection marker
- SapI-linearized pDAUGHTER vector:
- pMOTHER constructs: a yellow fluorescent protein gene (KringleYFP) was cloned into two pMOTHER vectors:
pDAUGHTER 441-SR (inducible T5 promoter, strong RBS, Kan resistance, pUC bacterial origin)
Methods:
- Reactions for the exchange of ORF from pMOTHER vectors to pDAUGHTER vector were set up in a single 1.5ml tube as described above. Reactions were incubated for 5, 10, 20, 40 or 60 minutes at room temperature.
- 2.5 µl of each reaction was transformed into 50 µl of NEB 10-beta competent E. coli and 100 µl of each transformation was plated onto LB Agar plates with 30 µg/mL kanamycin alone or with kanamycin and 100 µg/mL streptomycin or YEG (phenylalanine analog).
Results:
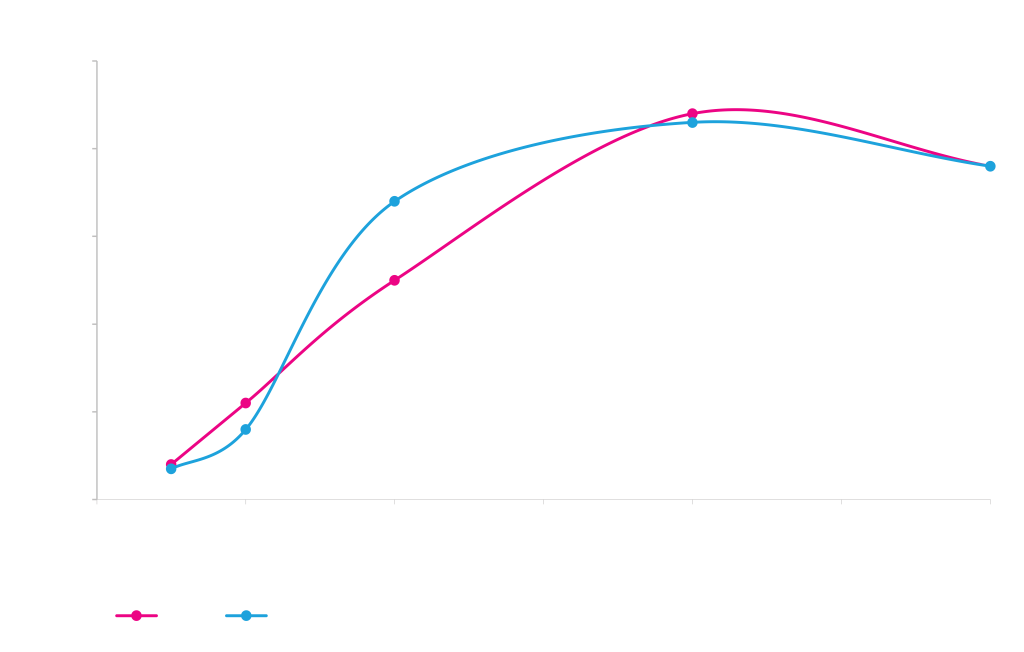
pM264-KringleYFP (rpsL/Amp) x pD441SR (high RBS/Kan)
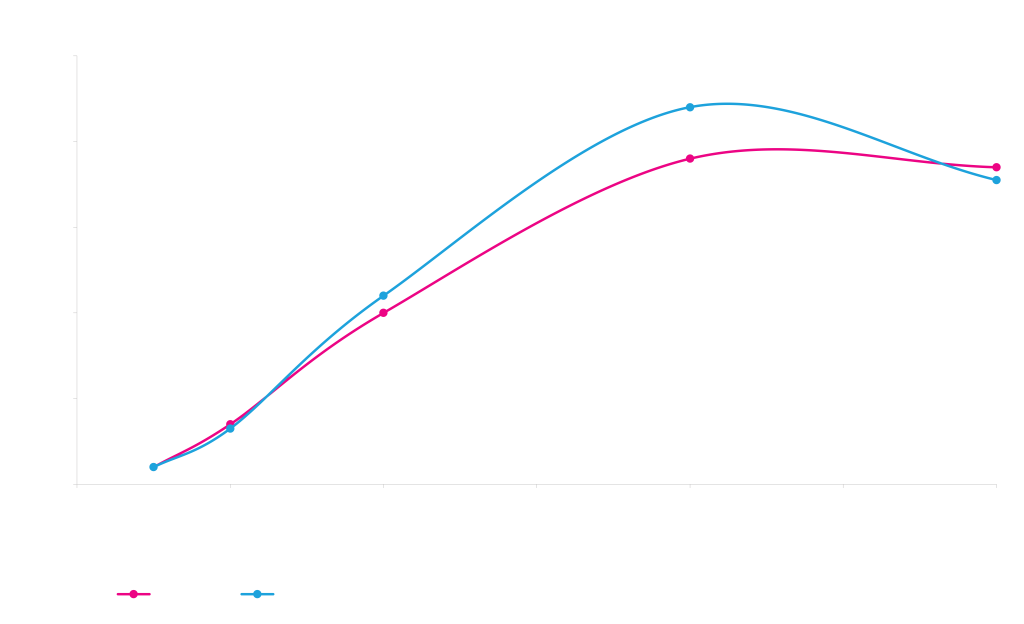
pM268-KringleYFP (pheS/Amp) x pD441SR (high RBS/Kan)
Electra system cloning time course with E. coli pDAUGHTER expression vectors. A gene encoding KringleYFP was cloned into an ampicillin-resistant pMOTHER vector with an rpsL counter-selection gene (pMOTHER264, left panel) or a PheS counter-selection gene (pMOTHER268, right panel). The pMOTHER vectors were mixed with a pre-linearized E. coli pDAUGHTER expression vector with inducible T5 promoter (pDAUGHTER441-SR) in the presence of SapI, T4 DNA ligase and ATP. Reaction mixtures were transformed into E. coli NEB 10-beta competent E. coli cells after various reaction times, and plated onto nutrient agar with kanamycin (blue lines) or kanamycin plus MOTHER counter-selection agent (red lines).
DAUGHTER constructs are selected because MOTHER and DAUGHTER vectors use different antibiotic resistance markers.
Since MOTHER and DAUGHTER are present in the transformation mixture, a small fraction (<5%) of the cells transformed with a DAUGHTER construct will also take up and maintain the MOTHER construct. MOTHER vectors therefore also carry a counter-selection marker, either rpsL (streptomycin sensitivity) or PheS (phenylalanine analog p-chlorophenylalanine sensitivity). Plating transformants onto media that contains both DAUGHTER selection antibiotic and MOTHER counter-selection agent reduces (rpsL) or completely eliminates (PheS) this small number of transformants that also carry the MOTHER.
Cloning from PCR products
To determine if it is possible to clone a PCR product without any prior treatment or cleanup, into a pDAUGHTER vector using the Electra one tube reaction approach described above.
Methods:
- A yellow fluorescent protein (KringleYFP) was amplified by PCR using primers:
- 107888A-ampF
- TACACGTACTTAGTCGCTGAAGGGGAAGTCTTCGCTCTTCTATGACGGCACTGACTGAAGGCGCAAAACTGTTCGAG
- 107888A-ampR
- AGGTACGAACTCGATTGACGTTTTTAGTCTTCGCTCTTCTACCTTAACGGTACGTTTCCAGGTCAACTGCCTTGATC
- 5 µl of each reaction was run on a 1% agarose-TBE gel. A strong and clean amplicon running at 750 bp was observed with an estimated concentration of 100ng/µl.
- Electra cloning of PCR product into a pDAUGHTER vector was carried out as a one tube reaction using 20 ng of linearized pDAUGHTER vector, 200 ng of PCR reaction and incubation time of 5 minutes at room temperature.
- 2.5 µl of each reaction was transformed into 50 µl of NEB 10-beta competent E. coli and 100 µl of each transformation was plated.
Results:
We observed thousands of yellow colonies and fewer than 5% white colonies (with no insert). The results demonstrate that crude PCR product can be cloned into a pDAUGHTER vector in 5 minutes at room temperature without any PCR reaction treatment or cleanup. It will therefore be possible to PCR amplify a gene of interest and efficiently clone the PCR product into any DAUGHTER vector. PCR allows for efficient transfer of an open reading frame of interest into a variety of host expression systems using the Electra cloning system and ATUM’s DAUGHTER expression vectors.
Electra Reagents Kit
The Electra Reagents Kit contains all necessary components to facilitate cloning a gene from a pMOTHER vector or a PCR product into an Electra pMOTHER or pDAUGHTER expression vector.
Learn more about the Electra Vector System.
Electra Reagents Kit Components
Electra Buffer Mix:
Supplied as a 10X mix, to be diluted to 1X in the final reaction mix.
Electra Enzyme Mix:
Supplied as a 20X mix, to be diluted to 1X in the final reaction mix. The mix contains SapI and T4 ligase, and is formulated for optimal cloning efficiency.
Positive Control:
A mix of pMOTHER vectors with a Tet promoter and a yellow fluorescent protein (KringleYFP) for Electra expression pDAUGHTER vectors, and a Tet promoter with a green fluorescent protein (DasherGFP) for Cas9 Electra pDAUGHTER vectors. It allows monitoring of the transfer of KringleYFP or DasherGFP into a pDAUGHTER expression vector and is seen as yellow or green colonies when plated with selection antibiotic.
FAQs
The Electra technology is covered by issued US patent 9,206,433 and related pending applications.
Webinar
SapI sites are removed in the cloning process: you will not be able to use SapI to remove your gene from the pDAUGHTER vector.
pD and pM vectors (without an ORF) are provided as linearized DNA in solution (10 reactions). pD or pM vectors with a control ORF are provided as circular plasmids (lyophilized).
Use the ATUM Bioinformatics Toolbox to facilitate primer analysis and design.
A PDF of the Electra protocol can be found here.
A PDF to make PheS or rpsL plates can be found here.