Enzymes
SPEED
Fast and easy gRNA design using ATUM’s design tool that minimizes off-target effects using ATUM’s scoring algorithms. ATUM will clone your gRNAs into the CRISPR construct of your choice and send you ready-to-transfect plasmid.
SPECIFICITY
Easy design of two tandem gRNAs for NickaseNinja vectors to enhance specificity.
PRECISION
The design tool enables suppression
of off-target effects.
Proteases
TEV protease is an engineered catalytic domain of the Tobacco Etch Virus Nla cysteine protease which is used to remove fusion tags from purified proteins. ATUM offers vectors with a TEV protease recognition site (ENLYFQ/G) between the tag and the ORF. The enzyme is His-tagged and affinity purified.
- Amount
- 1 mg affinity purified TEV-His protease in 50 mM Tris-HCl, pH 8, 150 mM NaCl, 40% glycerol, 1 mM DTT at 1 mg/ml.
- Activity
- TEV protease (100 µg/ml) shows 100% activity at 4°C overnight or 30°C for 1 hour in buffer with 50 mM Tris-HCl, pH 8, 150 mM NaCl and 1 mM DTT. While TEV protease cleaves both on and off-column, it is more efficient in solution (off-column).
- Properties
- A modified 27 kDa TEV protease construct containing a 6XHis tag for easy removal using His affinity media.
- Applications
- TEV protease is an efficient tool for fusion protein cleavage in solution or immobilized TEV protease on streptavidin-agarose.
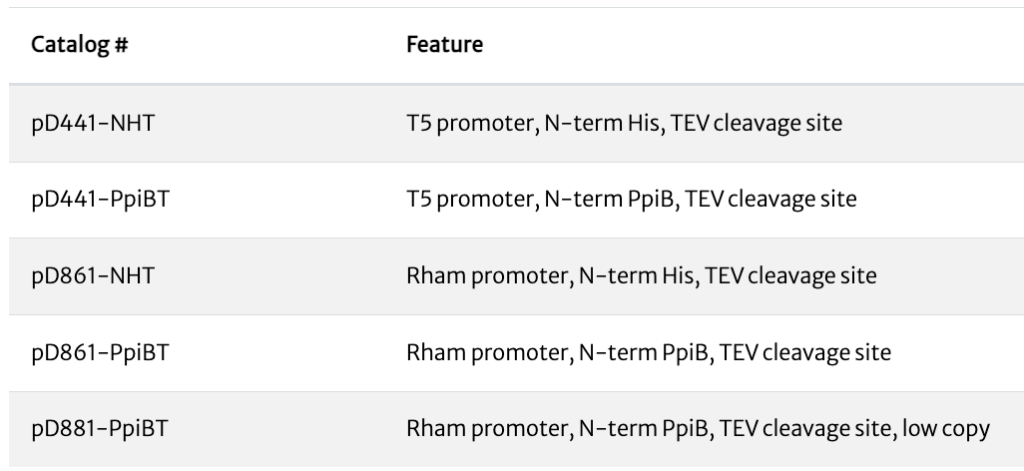
ATUM vectors with TEV cleavage sites
TEV Protease Cleavage Data
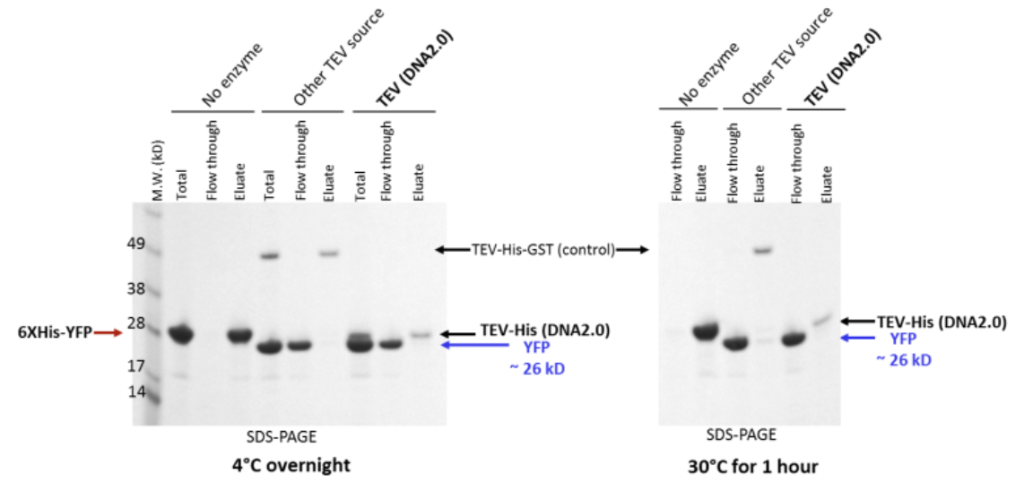
TEV protease cleaves at the TEV recognition site to efficiently remove the 6XHis Tag. Purification of KringleYFP and removal of 6XHis tag from the His-KringleYFP and TEV-His protease following cleavage with TEV-His protease is shown. KringleYFP with N-terminal 6XHis and TEV cleavage site ~28kD band (shown by red arrow) was cleaved with 100 µg/ml TEV protease at 4°C overnight or at 30°C for 1 hour. Commercially available TEV-His-GST protease was used for comparison; ‘no enzyme’ samples were run as negative controls. A SDS-PAGE gel was run with total (load), flow through and eluted fractions from an IMAC column and Coomassie stained. Total fraction shows KringleYFP and the TEV protease in samples treated with TEV protease (bands shown by black and blue arrows); flow through fractions showed only the His tag cleaved KringleYFP, seen as ~ 26kD band (shown by blue arrows) in TEV protease treated samples; no cleavage of KringleYFP-His was seen in the ‘no enzyme’ control. TEV protease cleaves efficiently at 4°C or 30°C. Although KringleYFP is stable at 30°C for fast TEV protease cleavage, for other proteins we recommend testing stability of your protein before incubation at 30°C.
TEV Protease Cleavage Process
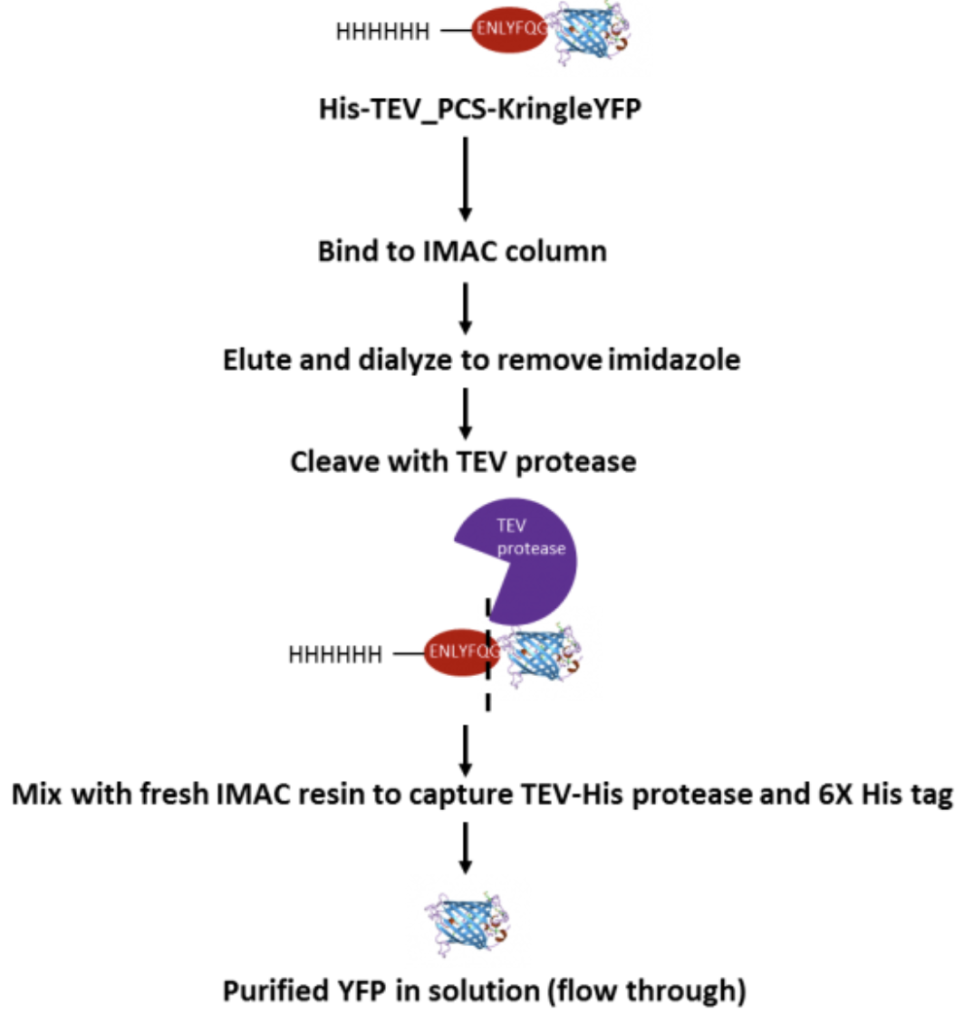
TEV Protease Cleavage Process. A general schematic for cleavage of the 6X His tag with TEV protease and purification is shown. KringleYFP was expressed with a N-terminal TEV protease cleavage site and a 6XHis tag (His-TEV_PCS-YFP). The 6XHis tag was cleaved in solution after the first IMAC (immobilized metal ion chromatography) column purification, dialysed into buffer (50 mM Tris-HCL, pH 8, 150 mM NaCl, 1 mM DTT) to remove imidazole and cut with 100 µg/ml TEV-His protease with mixing at 4°C overnight and at 30°C for 1hr. Samples were re-run on a IMAC column to remove His-tagged enzyme TEV-protease and the cleaved 6XHis tag from KringleYFP. The flow through has the purified tag-free protein.
HRV3C protease is a recombinant form of the human rhinovirus 3C cysteine protease which is used to remove fusion tags from purified proteins. ATUM offers vectors with a HRV3C protease recognition site (LEVLFQ/GP) between the tag and the ORF. The enzyme is GST-tagged and affinity purified.
Amount
1 mg affinity purified TEV-His protease in 50 mM Tris-HCl, pH 8, 150 mM NaCl, 40% glycerol, 1 mM DTT at 1 mg/ml
Activity
HRV3C protease at 125 ng/50 µg target protein shows 100% activity at 4°C overnight; 1 µg/50 µg target protein shows 100% activity at 4°C for 4 hours, cleavage was done in buffer with 50 mM Tris-HCl, pH 8, 100 mM NaCl, 0.5 mM EDTA, 10 mM reduced glutathione and 1 mM DTT. While HRV3C protease cleaves both on and off-column, it is more efficient in solution (off-column).
Properties
A recombinant 47 kDa HRV3C protease construct containing a GST tag for easy removal using Glutathione (GSH) resin.
Applications
HRV3C protease is an efficient tool for fusion protein cleavage in solution or immobilized HRV3C protease on streptavidin-agarose.
ATUM vectors with HRV3C cleavage sites
| Catalog# | Feature |
| pD441-GST | T5 promoter, N-term GST, HRV3C cleavage site |
| pD441-HMBP | T5 promoter, N-term HisMBP, HRV3C cleavage site |
HRV3C Protease Cleavage Data:
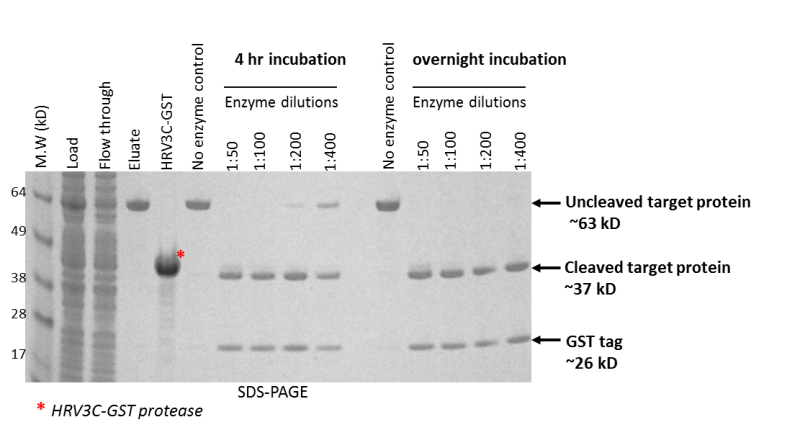
Cleavage of GST tag with HRV3C protease. The target protein, a RNA ligase with a HRV3C protease cleavage site (LEVLFQ/GP_PCS) and a GST tag, was purified on a Glutathione matrix. The eluted protein was incubated at 4°C for 4 hours and overnight with HRV3C-GST protease at various enzyme dilutions to determine the optimal enzyme concentration required to cleave the target. At 4 hours, a 1:100 ratio of enzyme to target protein (0.5 µg protease to 50 µg target protein) showed complete cleavage of the GST tag from the target. Complete cleavage was observed even at a 1:400 ratio of protease to target protein (125 ng protease to 50 µg of target protein).