Cell Line Development with Leap-In Transposase®
YIELD
Maximize your yield with ATUM’s Leap-In technology! Our proprietary platform generates highly productive stable pools, with pool titers very predictive of final clonal line titers.
STABILITY
Exceptional genetic stability leads to stable productivity of Leap-In-generated clonal cell lines. Derisk your workflow, from downstream process development to scale-up and manufacturing, with ATUM’s stable cell lines platform.
SPEED
Shave months off your CMC timeline with ATUM. With highly comparable quality and productivity between stable pools and derivative clones, get a head start on your analytical development, process development, and GLP toxicology studies.
EFFICIENCY
Cell line development is less labor intensive and does not require automation. No matter how small your team, many projects can be conducted simultaneously, from cell line development to cell line generation and cell line engineering.
ATUM’s Leap-In process includes consultation, research, and development of products for customers using genetic modification elements. These genetic modification elements often include transposases and transposons. ATUM’s Leap-In services further include analysis of DNA provided by customers for use in customers’ research efforts.
Leap-In Transposase®
Leap-In Transposases Integrate Intact DNA Constructs into Active Chromatin
By integrating single copies of the entire synthetic transposon into multiple transcriptionally-active genomic loci, ATUM’s Leap-In transposases will advance your cell line development workflow by:
- De-risking your workflow – By integrating the entire transposon, ATUM’s Leap-In transposases remove the risk of expression construct rearrangement or concatemerization, both common and problematic hallmarks of integration driven by random non-homologous recombination.
- Increasing Open Reading Frame stability – By maintaining structural integrity of the integrated sequence, ATUM’s Leap-In Transposase ensures that regulatory elements remain associated with the appropriate open reading frames, and that multiple open reading frames are in the desired expression ratio.
- Facilitating gene co-expression – Leap-In Transposase provides a very effective way to integrate genes that need to be expressed together as well as balancing respective gene expression in a controlled and optimal way, for example the heavy and light chains from monoclonal antibodies, or the three to four chains of bispecific antibodies.
Licensing available.
How Leap-In Transposase Works
01
An engineered transposon, comprising the expression construct and selection marker, flanked by inverted terminal repeats (ITRs).
02
Leap-In transposase recognizes its specific ITRs, binds and excises the transposon.
03
Leap-In transposase recognizes 4-base insertion sites across the host cell genome.
04
The intact transposon is inserted at up to 60 or more loci across the genome.
Expression Balancing and Optimization with VectorGPS®
Leap-In Transposon 2 ORFs
Leap-In Transposon 3 ORFs
Leap-In Transposon 4 ORFs
CELL LINE DEVELOPMENT WORKFLOW
Our Mammalian Hosts
ATUM currently uses two mammalian host cell lines with lineage traceability and documented provenance. All CHO cell lines are adapted to commercially-available chemically-defined serum-free formulations. No raw materials of animal origin are used during CHO cell line generation or in our cell line development process.
- DG44 from the lab of Dr. Lawrence Chasin (Columbia University)
- miCHO(R) (ATUM)
Stable Pool Selection
ATUM’s Leap-In transposase will increase the expression efficiency of your stable cell line. With most Leap-In transfected cells integrating multiple copies of the transposon into their genomes, ATUM’s technology ensures:
- Rapid recovery of stable pools consisting of many very similar cells.
- Highly comparable productivity and product quality of clones derived from these pools and the pools themselves.
- Early initiation of process and analytical development at just a few weeks post-transfection.
- Shorter overall CMC timelines due to the ability to prepare material for toxicology studies from stable pools.
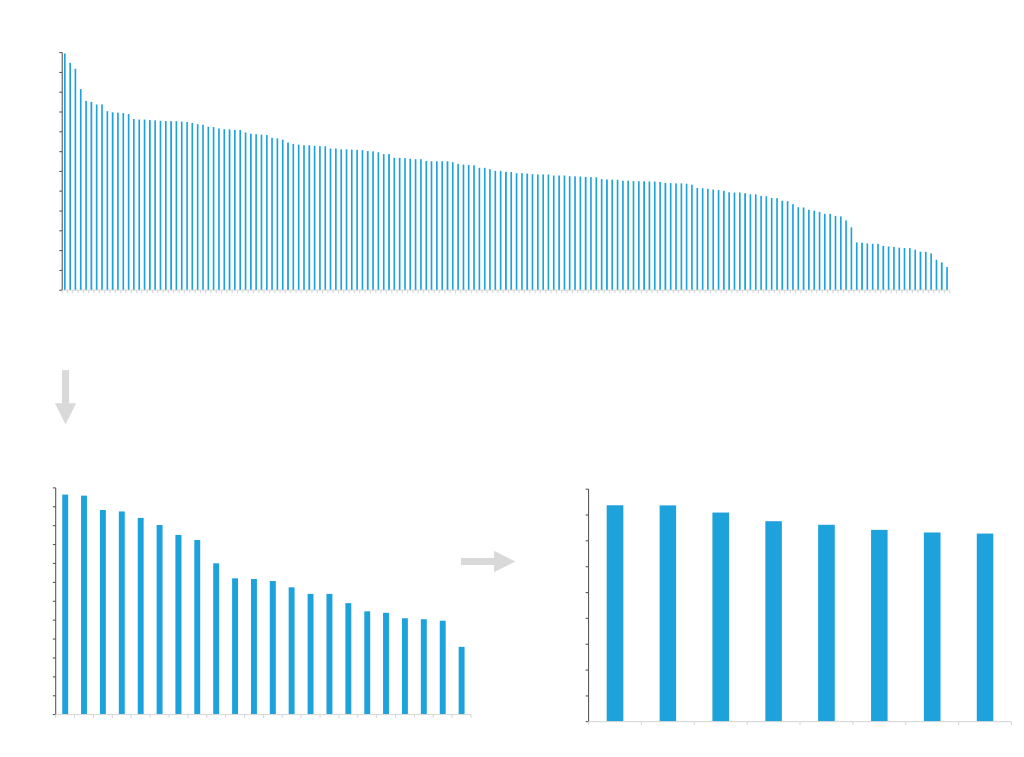
Clone Isolation
The clonal distribution within the stable pools generated by ATUM’s Leap-In technology are strongly biased towards high productivity. This reduces the number of clones that need to be tested to no more than a few hundred. Using Solentim VIPS™ seeding and clonal identification instruments, ATUM efficiently isolates high producing clones.
Stability Testing - Stable Expression Cell Lines
ATUM’s Leap-In transposases ensure your integration structures are stably maintained through an effective process:
- Leap-In Transposase mRNA is co-transfected with the synthetic transposon into the cell.
- The mRNA is translated and the Leap-In transposase protein transiently acts to integrate the transposon into the host genome.
- The transposase mRNA is degraded through the normal cellular RNA turnover pathways.
This means the cell can not generate endogenous transposase to mobilize the transposon and disrupt their stability. Because each integration contains a single copy of the transposon, there is no instability resulting from concatemer recombination or repeat-induced silencing. The number of integration could be as high as 60 which is a guarantee of high titers as the integration number of transposons correlates with protein yield.
Cell line construction Clonal cell lines are grown for 60 populations doublings then assayed for genetic stability by ddPCR, and productivity stability. The majority of clones created using Leap-In technologies exhibit no significant decreases in their volumetric productivity and integrated transposon copy number. Research cell banks (RCB) are established from the top performing clones. Cell banks are released after testing for sterility, mycoplasma, and transgene integrity by Southern blot and cDNA sequence analysis.
Licensing Leap-In Transposase
Bring the advantages of ATUM’s Leap-In technology to your in-house cell line development. The unique Leap-In transposase technology is covered by many issued and pending US patents, ensuring a broad integrated portfolio of related tools to maximize your success in cell line development and cell line engineering. The portfolio includes not only the Leap-In transposon/transposase itself but also complementing tools such as vector optimization, unique vector elements, codon optimization and more to provide you with our state-of-the-art bioengineering expertise. For the currently issued patents and related claims, please see our patents list.
We offer three convenient tiers of licensing to meet your research and commercialization needs. Under all licenses, Leap-In transposase mRNA and synthetic transposon DNA are purchased from ATUM. Your gene and vector designs are supported by our experienced Leap-In team and synthesized by ATUM to maximize your chance for success.
Platform licensing information can be found here.